[Physics FAQ] - [Copyright]
Original May 2003 by Don Koks.
What are Half Lives and Mean Lives?
Specifying the half life or mean life of a process is a way of quantifying
how fast it is occurring, when the whole process would in principle take forever
to complete. The example we will talk about here is radioactive growth and
decay, but examples from other fields include the recovery of a muscle after
some exertion, and the filling of a cistern.
In particular then, the half life of a radioactive element is the time
required for half of it to decay (i.e. change into another element, called the
"daughter" element).
So if a radioactive element has a half life of one hour, this means that half
of it will decay in one hour. After another hour, half of the remaining
material will decay. But why didn't all of that remaining
material decay in that second hour? Does the element somehow know that
it's decaying, and alter its decay speed to suit?
Textbooks are usually content with deriving of the law of decay, and don't
tend to address this question. And yet it forms a classic example of the
way in which research in physics (and science in general) is carried out.
Regardless of how we might expect an element to behave--where perhaps
the second half might be expected to decay in the same amount of time as the
first half--this simply does not happen. We must search for a theory that
predicts this.
Science is often thought to proceed by our logically deducing the laws that
govern the world. But it's not that simple; there are limits to what we
can deduce, especially about things in which we cannot directly
participate. Radioactive decay is a good example of this. We can't
use a microscope to watch the events that make an element decay. The
process is quite mysterious. But what we can do is make a simple theory of
how decay might work, and then use that theory to make a prediction of what
measurements we can expect. That's the way science proceeds: by making
theories that lead to predictions. Sometimes these predictions turn out to
be wrong. That's fine: it means we must tinker with the theory, perhaps
discard it outright, or maybe realise that it's completely okay under certain
limited circumstances. The hallmark of a good scientific theory is not
what it seems to explain, but rather what it predicts.
After all, a theory that says the universe just appeared yesterday, complete
with life on earth, fossils and so on, in a sense "explains" everything
beautifully by simply defining it to be so; but it predicts absolutely
nothing. So from a scientific point of view it is not a very useful
theory, because it contains nothing that allows its truth to be tested. On
the other hand, while it's arguable that the theory of quantum mechanics
explains anything at all, it certainly does predict a huge number of
different phenomena that have been observed; and that's what makes it a very
useful theory.
For radioactive decay, our theory is that the atoms decay, or change into
another atom, quite spontaneously. At a basic level, we don't know why
this should be; but we can only proceed step by step, and so first we begin with
this simple theory. We postulate that they decay independently of whether
their neighbours are decaying, and also that their tendency to decay is
independent of how old they are. A given atom might decay after one
microsecond, or one million years. However long it has been sitting intact
makes no difference to its ability to decay right now. If the mechanism
behind its decay is strong, in the sense that the atom has a large chance of
decaying, then it won't last long: after all, the chance that it won't
decay in some time interval is small, so the chance that it survives for any
appreciable amount of time is then also small. That can be worked out by
simple probability: multiplying together the probabilities that it doesn't decay
for a string of those time intervals. The statistics of decaying elements,
such as the mean and standard deviation of the number of atoms decaying in
various time intervals, were measured soon after radioactivity was discovered;
they were found to match those predicted by this idea of random decay, called
Poisson statistics. Whenever anything has a small chance of
happening, but there are lots of opportunities for it to happen, we get Poisson
statistics.
So certainly physics has not proven, and can never prove, that its
theory of atomic decay is true. The logical process is that if
atoms decay randomly, then Poisson statistics will result.
Experiment shows that Poisson statistics do indeed result, but logically this
does not mean that atoms decay randomly. Nevertheless, the way of science
is that we do postulate that atoms decay randomly, until a further experiment
calls this into question. But no experiment ever has. If this sounds
like a reverse use of logic, then consider the same ideas for mechanics.
Ideas of gravity, mass and acceleration were originally produced by Newton
through the same process: because they predicted planetary orbital periods that
could be verified experimentally. Because of this great success,
expressions such as F = ma and F = GMm/r2 came to
be canonical in physics. The logic was indeed being used in reverse; but
no one was surprised when, three centuries later, one of the moon astronauts
dropped a feather and a hammer together in the moon's vacuum, and found that
they both fell at the same rate (although it was still beautiful and dramatic to
watch!). That reverse logic had, after all, allowed him to get to the moon
in the first place. So this way of conducting science works very well.
A "people" experiment to simulate radioactive decay
We can perform a simple test of this theory of radioactive decay by doing an
experiment with a big group of people; the group should be large so as to get
good statistics. Put 1000 people into a big hall, and give each a
coin. Each person represents a radioactive atom, and the coin represents
their ability to decay. Tell everybody to toss their coin once per
minute. If the result is heads, then the person should immediately leave
the hall (which corresponds to the atom decaying). If tails, then take no
action, except waiting for another minute to elapse, and then tossing again.
What happens? After one minute, roughly half of the people get up and
walk out, because their coins landed heads up. After another minute,
everyone tosses again, and about half the remainder will walk out. Of
course we don't expect everyone to leave after the second minute; as
each minute goes by, roughly half of the group walks out. Our simple model
of random behaviour has produced a half life! We say that this particular
"element" has a half life of one minute. Really of course, the laws of
physics don't operate at one minute intervals; we should really ask everyone to
toss continuously. This is fine, but it would cause a lot of commotion and
make it harder to see what was happening.
Although it's not obvious, it can be shown that in such a situation where the
amount of an element halves in a constant time interval, it will also "third" in
a different constant time interval that is not hard to calculate. In fact,
we can choose any number we like to describe the decay speed. Choosing the
halving time is conventional because it's unambiguous. If a radioactive
element was said to have a "third life" of one hour, then no one would ever
remember if that means that after one hour one third has decayed, or
one third is left over. Specifying the half life doesn't produce
this problem of course.
Getting back to our group of 1000 people, still tossing every minute, we
could change its half life by changing the requirement to leave. For
example, every minute each person tosses their coin quickly twice. But
now, only those tossing two heads must leave. In that case, after the
first minute, one quarter will leave, and after the second minute, one quarter
of the remainder will leave. The half life now is longer than one
minute. So, we've arranged for this "element" to decay more slowly, by
lowering the chance that any one of its constituent atoms decays. Remember
that any particular person who sits and tosses their coin, might sit for any
amount of time, irrespective of how many of their neighbours have now left the
hall. They might even sit for years, tossing and tossing, and always
finding their coin lands tails up. The chance is small of course that this
will happen, but it just might. The chance that any person leaves is
completely independent of how long they have been sitting there.
A "mustard seed" experiment to simulate radioactive decay
Here is another way of demonstrating radioactive decay, this time closer to
the feel of a real experiment. Put a couple of teaspoons of mustard seeds
in a mortar, and grind them with a pestle. For a classroom demonstration,
have a microphone nearby that amplifies the click as each seed bursts. In
the random crashing-together of the seeds, the chance that any particular one
will burst is roughly independent of its neighbours and also roughly independent
of time. So what we hear is initially a burst of clicks that soon dies
down, with a half life of several seconds or longer. This is just like
what would be heard if we put a Geiger counter next to a rapidly decaying
element.
The mean life of a process
Given our radioactive element, if half of its atoms have decayed after one
half life, then we can expect there to be some kind of well defined average life
expectancy: the mean life, for the atoms, which is somewhat longer than
the half life. It turns out that the mean life equals the half life
divided by the natural logarithm of 2 (about 0.693). The mean life also
turns out to exactly equal the number tau that appears in the
exponential term e-t/tau involved with describing decay or
growth, called the time constant. The label "time constant" is
just a name for tau; the fact that the mean life happens to equal
tau is a kind of neat coincidence that allows us to think of the mean
life in the following, alternative, way.
We said above that the speed of any decay process can be described by the
time it takes for the remaining amount of the element to fall to a half, or a
third, or any fraction at all. One special choice of that number is
e, and the length of time after which the amount remaining has been
reduced to 1/e of the original also happens to equal the mean life of
the decay. This is one reason why the number e is so
special. Besides the fact that e has friendly properties that
simplify the mathematics of growth and decay, it also quantifies such an
everyday idea as the average life expectancy of the atoms.
When we plot the amount of a radioactive element as a function of time, we
find that it drops with a characteristic "exponential decay curve" that helps to
show this half life idea more mathematically. Here is a question: 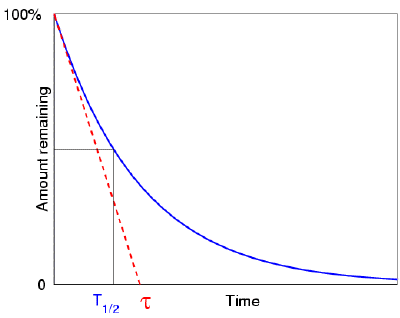 suppose that the element did not decay exponentially (i.e.
with a half life), but instead decayed linearly, meaning its rate of decay was
always equal to its initial rate (giving rise to a simple "first half in one
minute and the second half in the next minute--and then, all gone" sort of
idea). In such an idealised case, how long would it take to completely
vanish? That is, suppose we have 1000 atoms of a radioactive element, and
initially there are 10 atoms decaying per second. If it was to
keep decaying at this rate (it won't, but imagine that it did), then how long
would it take to completely vanish? The answer is of course 100
seconds. Now it turns out that this is precisely equal to the mean life of
the real element. (Which means that we know straight away that the real
element's half life is 69.3 seconds.)
suppose that the element did not decay exponentially (i.e.
with a half life), but instead decayed linearly, meaning its rate of decay was
always equal to its initial rate (giving rise to a simple "first half in one
minute and the second half in the next minute--and then, all gone" sort of
idea). In such an idealised case, how long would it take to completely
vanish? That is, suppose we have 1000 atoms of a radioactive element, and
initially there are 10 atoms decaying per second. If it was to
keep decaying at this rate (it won't, but imagine that it did), then how long
would it take to completely vanish? The answer is of course 100
seconds. Now it turns out that this is precisely equal to the mean life of
the real element. (Which means that we know straight away that the real
element's half life is 69.3 seconds.)
This way of looking at the concept of mean life also appeals to our
intuition: it says that if the atoms behaved in the nice, linear way
that we humans love to think in, then the time needed for them all to decay
would be exactly equal to the mean life of the actual, real world,
element. You can see that the number e is tied in to simple ideas
of linearity, and that's another reason why it is such a powerful number in
mathematical analysis.
This same idea applies to the growth of the daughter element. Suppose
for simplicity that the daughter is not itself radioactive. Initially
there are no daughter atoms, and gradually they grow as the parent element
decays. Their growth must eventually flatten out, and this takes infinite
time, for the same reason that it takes infinite time for the parent element to
fully decay. We ask the question: if the growth of the daughter
continued at its initial rate, (which it doesn't, remember!), then how long
would it take for the sample to be completely composed of daughter atoms?
Again, this time interval turns out to be the mean life of the parent.
The mean life and "probability per second"
That last way of applying the mean life to growth produces a way of speaking
that is not always well understood by those who use it. Suppose we have a
single atom of a radioactive element, sitting in front of us, waiting to
decay. We ask: what is the probability that after some time t, it
has decayed? On the average, we can expect that after one mean life
tau has elapsed, there's a reasonable chance that the atom will have
decayed. Although it might take a million times that period before it
actually does decay, we feel confident that after perhaps two or three mean
lives have passed, the atom will almost certainly have decayed.
We cannot really plot this probability as a function of time, because that
would require knowing when the atom will decay (since then the probability
equals one). So, the best we can do is to make a generic plot that
correctly describes the whole population of decaying atoms, since the atom in
front of us behaves just like the other atoms of the element. In that
case, we expect that the probability will grow, tending towards one as
t goes to infinity. Remember: this is an average sort of
prediction; any individual atom will certainly attain one at some point.
But it's the best we can do.
The actual expression for this probability turns out to be 1 -
e-t/tau, which indeed tends toward one as t tends to
infinity. (It's actually the same curve as that describing the growth of
the daughter element.) 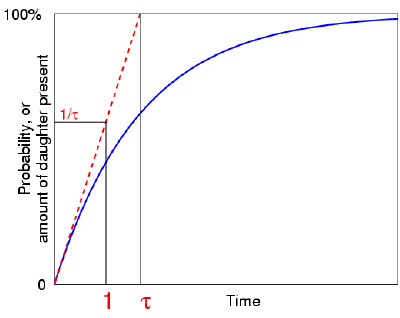 Now,
what is the value of this (generic) probability when t=1: what is the
chance that the atom has decayed after one unit of time? Well, the answer
is 1 - e-1/tau. . . which might not communicate very
much! So as we did before, let's ask another, related, question.
If the probability of decaying within time t continued to
increase at its initial rate, (which it doesn't really!), then how long would it
take for the atom to decay--after how long would this probability reach
one? Again, this time interval turns out to be the mean life
tau. But if our linear, simplified, atom reaches decay
probability one after a time tau, then it must reach probability
1/tau after a unit time.
Now,
what is the value of this (generic) probability when t=1: what is the
chance that the atom has decayed after one unit of time? Well, the answer
is 1 - e-1/tau. . . which might not communicate very
much! So as we did before, let's ask another, related, question.
If the probability of decaying within time t continued to
increase at its initial rate, (which it doesn't really!), then how long would it
take for the atom to decay--after how long would this probability reach
one? Again, this time interval turns out to be the mean life
tau. But if our linear, simplified, atom reaches decay
probability one after a time tau, then it must reach probability
1/tau after a unit time.
In our example above with 1000 atoms and initially 10 decays per second, we
concluded the mean life was tau = 100 seconds. So, if
the chance that any particular atom had decayed was to keep increasing uniformly
at its initial rate, then after one second, the chance that it had decayed would
be 1/100. This makes sense: 1/100 of the initial 1000 atoms is the 10
atoms we measured to have decayed. So we say "the atom has a probability
of decay of 1/100 per second". Remember, this doesn't mean that after 100
seconds it will definitely have decayed! This would only be true if the
atom were to behave in a simple, linear, way.
The fact that the atom's probability of having decayed is "slowing down", is
just like a pushed trolley that starts out with a speed of 1/100 metre per
second, but decelerating due to friction at just the right rate so as to match
our atom. If there were no friction so that it moved at constant speed,
then it would take 100 seconds to travel a metre. It doesn't do this of
course--it never quite reaches a metre distance because it continuously slows
down; but 1/100 m/s refers to its initial rate of distance
increase. And likewise for our generic atom that represents the whole
population of atoms, "1/100 per second" refers to its initial rate of
"decay-probability increase". In an average sense for the whole
population, that decay probability will never quite reach one, although it
will eventually reach one for any particular atom.
This whole discussion of growth can also be applied to the daughter element,
because that grows with precisely the same time constant tau. If
it continued to grow at its initial rate, then after a time tau, the
sample would be completely composed of daughter atoms.
You can see that we can juggle these numbers and get a feel for the details
of an element's decay or growth. But for these more refined ideas, it's
certainly a whole lot easier to think in terms of the mean life, than the half
life. Even so, both the mean life and the half life give us an intuitive
feel for radioactive decay, and each has its own domain of usefulness.
 suppose that the element did not decay exponentially (i.e.
with a half life), but instead decayed linearly, meaning its rate of decay was
always equal to its initial rate (giving rise to a simple "first half in one
minute and the second half in the next minute--and then, all gone" sort of
idea). In such an idealised case, how long would it take to completely
vanish? That is, suppose we have 1000 atoms of a radioactive element, and
initially there are 10 atoms decaying per second. If it was to
keep decaying at this rate (it won't, but imagine that it did), then how long
would it take to completely vanish? The answer is of course 100
seconds. Now it turns out that this is precisely equal to the mean life of
the real element. (Which means that we know straight away that the real
element's half life is 69.3 seconds.)
suppose that the element did not decay exponentially (i.e.
with a half life), but instead decayed linearly, meaning its rate of decay was
always equal to its initial rate (giving rise to a simple "first half in one
minute and the second half in the next minute--and then, all gone" sort of
idea). In such an idealised case, how long would it take to completely
vanish? That is, suppose we have 1000 atoms of a radioactive element, and
initially there are 10 atoms decaying per second. If it was to
keep decaying at this rate (it won't, but imagine that it did), then how long
would it take to completely vanish? The answer is of course 100
seconds. Now it turns out that this is precisely equal to the mean life of
the real element. (Which means that we know straight away that the real
element's half life is 69.3 seconds.) Now,
what is the value of this (generic) probability when t=1: what is the
chance that the atom has decayed after one unit of time? Well, the answer
is 1 - e-1/tau. . . which might not communicate very
much! So as we did before, let's ask another, related, question.
If the probability of decaying within time t continued to
increase at its initial rate, (which it doesn't really!), then how long would it
take for the atom to decay--after how long would this probability reach
one? Again, this time interval turns out to be the mean life
tau. But if our linear, simplified, atom reaches decay
probability one after a time tau, then it must reach probability
1/tau after a unit time.
Now,
what is the value of this (generic) probability when t=1: what is the
chance that the atom has decayed after one unit of time? Well, the answer
is 1 - e-1/tau. . . which might not communicate very
much! So as we did before, let's ask another, related, question.
If the probability of decaying within time t continued to
increase at its initial rate, (which it doesn't really!), then how long would it
take for the atom to decay--after how long would this probability reach
one? Again, this time interval turns out to be the mean life
tau. But if our linear, simplified, atom reaches decay
probability one after a time tau, then it must reach probability
1/tau after a unit time.